Aortic dissection
BASIC INFORMATION
Aortic dissection results from a tear in the intima, such that blood penetrates between the intimal layer and the media of the aortic wall. This creates a false lumen in the arterial wall. Typically a second tear of the intima creates the exit for the blood. This exit site can be located in the ascending aorta, in the descending aorta, or the iliac vessels. The Stanford classification divides aortic dissections into two types:
* type A: all dissections involving the ascending aorta, regardless of the site of origin
* type B: all dissections not involving the ascending aorta.
SYNONYMS
Dissecting aortic aneurysm, unspecified site
EPIDEMIOLOGY & DEMOGRAPHICS
PREDOMINANT SEX: Males > females
PEAK INCIDENCE: Sixth and seventh decades
RISK FACTORS: Hypertension
CLASSIFICATION
• Stanford type A (includes DeBakey types I and II) involves ascending aorta
• Stanford type B (DeBakey type III) limited to descending aorta
PHYSICAL FINDINGS & CLINICAL PRESENTATION
Clinical Features. The patient usually complains of a severe “tearing” chest pain, which may originate in the back or radiate from the precordium to the back. The localization of the pain may hint towards the origin of the dissection. A retrosternal pain radiating to the back suggests a dissection in the ascending aorta. An interscapular pain suggests a dissection in the descending aorta, and an abdominal pain may hint at a dissection in the abdominal aorta. Pain onset is typically abrupt and persistent, and collapse is common. The sudden onset and the lack of ECG changes help to differentiate between an aortic dissection and a myocardial infarction. The following additional symptoms may be associated with aortic dissection:
* Dissection of the carotid arteries may result in cerebral hypoperfusion with neurologic symptoms (e. g. hemiplegia).
* In about half of the patients with aortic dissection, shape changes of the aortic root result in acute aortic insufficiency.
* Compression of the truncus brachiocephalicus results in a pulse deficit and blood pressure difference between right and left arm. Such a pulse deficit is almost pathognomonic for aortic dissection, since only arterial emboli or Takayasu disease can cause a similar sign.

Fig. 5.21 Aortic dissection of the ascending aorta (type A) in a 57-year-old patient.
a Clearly visible is the grotesquely dilated ascending aorta (arrows).
b Computed tomography in the same patient shows the aneurysm and the dissection membrane (arrows). A = aneurysm, AP = pulmonary artery, Ao = aorta descendens, * = true lumen of the ascending aorta.
a Clearly visible is the grotesquely dilated ascending aorta (arrows).
b Computed tomography in the same patient shows the aneurysm and the dissection membrane (arrows). A = aneurysm, AP = pulmonary artery, Ao = aorta descendens, * = true lumen of the ascending aorta.
* Other less frequent complications are the compression of the coronary arteries resulting in myocardial infarction, rupture of the dissection into the pericardial space with pericardial tamponade, hematothorax, rupture of the descending aorta with bleeding into the mediastinum or abdomen, a compression of the renal arteries with subsequent acute renal failure, mesenteric infarction with an acute abdomen due to compression of celiac and/or superior mesenteric artery, and acute limb ischemia secondary to obstruction of the flow in the arteria iliaca.
• Abrupt onset of severe chest pain
• May present with back and/or abdominal pain
• 12% of patients present with syncope
• Hypertension or hypotension
• Unequal or absent pulses
• Murmur of aortic insufficiency possible
• Neurologic abnormalities caused by carotid obstruction (hemiplegia) or spinal cord ischemia (paraplegia)
• Mass effect can cause Horner’s syndrome, superior vena cava syndrome, hoarseness, dysphagia, airway compromise
• Cardiac tamponade caused by dissection into pericardial sac
• Abrupt onset of severe chest pain
• May present with back and/or abdominal pain
• 12% of patients present with syncope
• Hypertension or hypotension
• Unequal or absent pulses
• Murmur of aortic insufficiency possible
• Neurologic abnormalities caused by carotid obstruction (hemiplegia) or spinal cord ischemia (paraplegia)
• Mass effect can cause Horner’s syndrome, superior vena cava syndrome, hoarseness, dysphagia, airway compromise
• Cardiac tamponade caused by dissection into pericardial sac
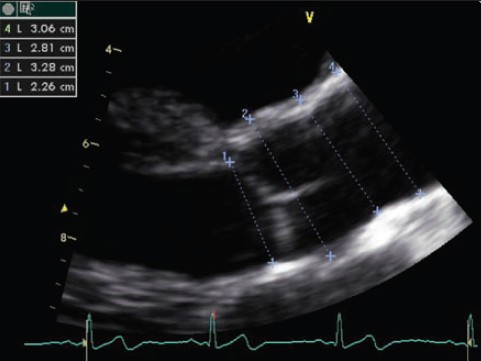
2-D Longitudinal echocardiogram of the aortic root showing measurements of aortic size.
DIAGNOSIS
Aortic dissection must be suspected in patients presenting with typical symptoms and signs and pathologic chest radiograph (Fig. 5.21a). Diagnosis is confirmed by transesophageal echocardiography or computed tomography (Fig. 5.21b) or MR-angiography.
• Acute MI
• Aortic insufficiency
• Nondissecting aortic aneurysm
• Musculoskeletal pain
• Pericarditis
• Angina
• Cholecystitis
WORKUP
Physical examination and imaging
LABORATORY TESTS
ECG may suggest LVH or pericardial effusion.
IMAGING STUDIES
• Chest x-ray may show widened mediastinum (62%), displacement of aortic intimal calcium
• Transesophageal echocardiography, sensitivity 97% to 100%, can detect aortic insufficiency and pericardial effusion; study of choice in many centers
• MRI, sensitivity 90% to 100%, difficult for unstable intubated patients
• CT, sensitivity 83% to 100%, involves IV contrast, cannot detect aortic insufficiency
• Aortography, sensitivity 81% to 91%, involves IV contrast, allows visualization of coronary arteries
TREATMENT
ACUTE GENERAL Rx
• Admit to ICU for hemodynamic monitoring.
• Decrease contractility and BP using b-blocker IV; labetalol 20 mg initially, then 40 to 80 mg q10min, titrate to systolic BP 120 mm Hg and pulse 60.
• Decrease BP using sodium nitroprusside by IV infusion pump 0.3 to 10 mg/kg/min, titrate to systolic BP 120 mm Hg.
• Type A (proximal) dissections usually require emergent or urgent surgery.
• Percutaneous fenestration and/or stent placement for selected patients
• Type B (distal) dissection, if stable, can be treated medically and monitored for signs of propagation, branch compromise, continued pain, or impending rupture.
DISPOSITION
• Untreated, mortality is 85% within 2 wk.
• Type A dissections surgically treated, mortality is 15% to 20%.
• Type B dissections treated medically, mortality is 15% to 20%.
Aortic dissection must be suspected in patients presenting with typical symptoms and signs and pathologic chest radiograph (Fig. 5.21a). Diagnosis is confirmed by transesophageal echocardiography or computed tomography (Fig. 5.21b) or MR-angiography.
• Acute MI
• Aortic insufficiency
• Nondissecting aortic aneurysm
• Musculoskeletal pain
• Pericarditis
• Angina
• Cholecystitis
WORKUP
Physical examination and imaging
LABORATORY TESTS
ECG may suggest LVH or pericardial effusion.
IMAGING STUDIES
• Chest x-ray may show widened mediastinum (62%), displacement of aortic intimal calcium
• Transesophageal echocardiography, sensitivity 97% to 100%, can detect aortic insufficiency and pericardial effusion; study of choice in many centers
• MRI, sensitivity 90% to 100%, difficult for unstable intubated patients
• CT, sensitivity 83% to 100%, involves IV contrast, cannot detect aortic insufficiency
• Aortography, sensitivity 81% to 91%, involves IV contrast, allows visualization of coronary arteries
TREATMENT
ACUTE GENERAL Rx
• Admit to ICU for hemodynamic monitoring.
• Decrease contractility and BP using b-blocker IV; labetalol 20 mg initially, then 40 to 80 mg q10min, titrate to systolic BP 120 mm Hg and pulse 60.
• Decrease BP using sodium nitroprusside by IV infusion pump 0.3 to 10 mg/kg/min, titrate to systolic BP 120 mm Hg.
• Type A (proximal) dissections usually require emergent or urgent surgery.
• Percutaneous fenestration and/or stent placement for selected patients
• Type B (distal) dissection, if stable, can be treated medically and monitored for signs of propagation, branch compromise, continued pain, or impending rupture.
DISPOSITION
• Untreated, mortality is 85% within 2 wk.
• Type A dissections surgically treated, mortality is 15% to 20%.
• Type B dissections treated medically, mortality is 15% to 20%.
ETIOLOGY
Aortic dissection occurs most commonly in the sixth or seventh decade of life. Hypertension, which is present in 80% of the patients, is a predisposing factor. It is believed that hypertension contributes to medial degeneration by compression of the vasa vasorum of the aorta. Atherosclerosis of the aorta and, in some cases, previous surgery of the aorta (e. g. coronary bypass operation or aortic valve replacement) may contribute to the pathogenesis of aortic dissection. Aortic dissection may, however, occur in younger patients. In these cases aortic diseases with medial degeneration are considered the main predisposing factors. The most important disease causing aortic dissection is Marfan syndrome. Other diseases include Ehlers-Danlos syndrome, coarctation of the aorta, or fibromuscular dysplasia. In younger women, about 50% of cases of aortic dissection occurs during pregnancy. If a patient with Marfan syndrome becomes pregnant, there is a high likelihood of aortic dissection, typically occurring in the third trimester. Aortic dissections can also be caused by trauma or iatrogenic injuries, by catheters, or an intraaortic balloon pump.
• Hypertension
• Cystic medial necrosis
• Marfan’s syndrome
• Congenital aortic valve abnormalities
• Previous cardiac surgery
Aortic dissection occurs most commonly in the sixth or seventh decade of life. Hypertension, which is present in 80% of the patients, is a predisposing factor. It is believed that hypertension contributes to medial degeneration by compression of the vasa vasorum of the aorta. Atherosclerosis of the aorta and, in some cases, previous surgery of the aorta (e. g. coronary bypass operation or aortic valve replacement) may contribute to the pathogenesis of aortic dissection. Aortic dissection may, however, occur in younger patients. In these cases aortic diseases with medial degeneration are considered the main predisposing factors. The most important disease causing aortic dissection is Marfan syndrome. Other diseases include Ehlers-Danlos syndrome, coarctation of the aorta, or fibromuscular dysplasia. In younger women, about 50% of cases of aortic dissection occurs during pregnancy. If a patient with Marfan syndrome becomes pregnant, there is a high likelihood of aortic dissection, typically occurring in the third trimester. Aortic dissections can also be caused by trauma or iatrogenic injuries, by catheters, or an intraaortic balloon pump.
• Hypertension
• Cystic medial necrosis
• Marfan’s syndrome
• Congenital aortic valve abnormalities
• Previous cardiac surgery
Contacts: lubopitno_bg@abv.bg www.encyclopedia.lubopitko-bg.com Corporation. All rights reserved.
DON'T FORGET - KNOWLEDGE IS EVERYTHING!