Basic Chemistry
Matter is anything that takes up space and has weight; it can be a solid, a liquid, or a gas. Therefore, not only are we humans matter, but so are the water we drink and the air we breathe.
Elements and Atoms
All matter is composed of basic substances called elements. It’s quite remarkable that there are only 92 naturally occurring elements. It is even more surprising that over 90% of the human body is composed of just four elements: carbon, nitrogen, oxygen, and hydrogen. Every element has a name and a symbol; for example, carbon has been assigned the atomic symbol C (Fig. 2.1a). Some of the symbols we use for elements are derived from Latin. For example, the symbol for sodium is Na because natrium in Latin means sodium. Elements are composed of tiny particles called atoms. The same name is given to both an element and its atoms.
Atoms
An atom is the smallest unit of an element that still retains the chemical and physical properties of the element. Although it is possible to split an atom by physical means, an atom is the smallest unit to enter into chemical reactions. For our purposes, it is satisfactory to think of each atom as having a central nucleus and pathways about the nucleus called shells. The subatomic particles called protons and neutrons are located in the nucleus, and electrons orbit about the nucleus in the shells (Fig. 2.1b). Most of an atom is empty space. If we could draw an atom the size of a football stadium, the nucleus would be like a gumball in the center of the field, and the electrons would be tiny specks whirling about in the upper stands. Protons carry a positive (+) charge, and electrons have a negative (-) charge. The atomic number of an atom tells you how many protons, and therefore how many electrons, an atom has when it is electrically neutral. For example, the atomic number of carbon is six; therefore, when carbon is neutral, it has six protons and six electrons. How many electrons are in each shell of an atom? The inner shell is the lowest energy level and can hold only two electrons; after that, each shell, for the atoms noted in Figure 2.1a, can hold up to eight electrons. Using this information, we can calculate that carbon has two shells and that the outer shell has four electrons. The number of electrons in the outer shell determines the chemical properties of an atom, including how readily it enters into chemical reactions. As we shall see, an atom is most stable when the outer shell has eight electrons. (Hydrogen, with only one shell, is an exception to this statement. Atoms with only one shell are stable when this shell contains two electrons.) The subatomic particles are so light that their weight is indicated by special designations called atomic mass units. Protons and neutrons each have a weight of one atomic mass unit, and electrons have almost no mass. Therefore, the atomic weight of an atom generally tells you the number of protons plus the number of neutrons. How could you calculate that carbon (C) has six neutrons? Carbon’s atomic weight is 12, and you know from its atomic number that it has six protons. Therefore, carbon has six neutrons (Fig. 2.1b). Also, as shown in Figure 2.1b, the atomic number of an atom is often written as a subscript to the lower left of the atomic symbol. The atomic weight is often written as a superscript to the upper left of the atomic symbol.
Figure 2.1 Elements and atoms. a. The atomic symbol, number, and weight are given for common elements in the body. b. The structure of carbon shows that an atom contains the subatomic particles called protons (p) and neutrons (n) in the nucleus (colored pink) and electrons (colored blue) in shells about the nucleus.

Isotopes
Isotopes of the same type of atom differ in the number of neutrons and therefore in weight. For example, the element carbon has three common isotopes:
Carbon 12 has six neutrons, carbon 13 has seven neutrons, and carbon 14 has eight neutrons. Unlike the other two isotopes of carbon, carbon 14 is unstable and breaks down over time. As carbon 14 decays, it releases various types of energy in the form of rays and subatomic particles, and therefore it is a radioactive isotope. The radiation given off by radioactive isotopes can be detected in various ways. You may be familiar with the use of a Geiger counter to detect radiation.
Isotopes of the same type of atom differ in the number of neutrons and therefore in weight. For example, the element carbon has three common isotopes:
Carbon 12 has six neutrons, carbon 13 has seven neutrons, and carbon 14 has eight neutrons. Unlike the other two isotopes of carbon, carbon 14 is unstable and breaks down over time. As carbon 14 decays, it releases various types of energy in the form of rays and subatomic particles, and therefore it is a radioactive isotope. The radiation given off by radioactive isotopes can be detected in various ways. You may be familiar with the use of a Geiger counter to detect radiation.

Low Levels of Radiation
The importance of chemistry to biology and medicine is nowhere more evident than in the many uses of radioactive isotopes. A radioactive isotope behaves the same as do the stable isotopes of an element. This means that you can put a small amount of radioactive isotope in a sample, and it becomes a tracer by which to detect molecular changes. Specific tracers are used in imaging the body’s organs and tissues. For example, after a patient drinks a solution containing a minute amount of radioactive iodine (131I), the tracer becomes concentrated in the thyroid, which takes it up to make the hormone thyroxine. (No other organ takes up 131I.) A subsequent image of the thyroid indicates whether it is healthy in structure and function (Fig. 2.2). Positron-emission tomography (PET) is a way to determine the comparative activity of tissues.
The importance of chemistry to biology and medicine is nowhere more evident than in the many uses of radioactive isotopes. A radioactive isotope behaves the same as do the stable isotopes of an element. This means that you can put a small amount of radioactive isotope in a sample, and it becomes a tracer by which to detect molecular changes. Specific tracers are used in imaging the body’s organs and tissues. For example, after a patient drinks a solution containing a minute amount of radioactive iodine (131I), the tracer becomes concentrated in the thyroid, which takes it up to make the hormone thyroxine. (No other organ takes up 131I.) A subsequent image of the thyroid indicates whether it is healthy in structure and function (Fig. 2.2). Positron-emission tomography (PET) is a way to determine the comparative activity of tissues.
Radioactively labeled glucose emits a subatomic particle known as a positron. When labeled glucose is injected into the body. The radiation given off is detected by sensors and analyzed by a computer. The result is a color image that shows which tissues took up glucose and are metabolically active (Fig. 2.3). A PET scan of the brain can help diagnose a brain tumor, Alzheimer disease, epilepsy, or stroke.
Figure 2.2 Use of radiation to aid a diagnosis. After the administration of radioactive iodine, a scan of the thyroid reveals pathology. The missing portion of the gland is cancerous and therefore failed to take up the iodine.

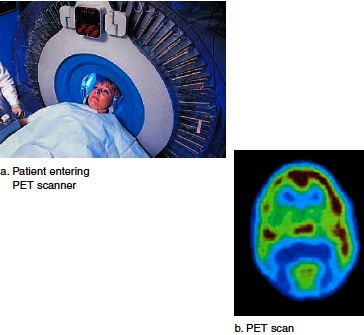
Figure 2.3 Use of radiation to study the brain. After the administration of radioactively labeled glucose, a PET scan reveals which portions of the brain are most active.
High Levels of Radiation
Radioactive substances in the environment can harm cells, damage DNA, and cause cancer. The release of radioactive particles following a nuclear power plant accident can have far-reaching and long-lasting effects on human health. The harmful effects of radiation can also be put to good use, however. Radiation from radioactive isotopes has been used for many years to sterilize medical and dental products. Now the possibility exists that it can be used to sterilize the U.S. mail to free it of possible pathogens, such as anthrax spores. The ability of radiation to kill cells is often applied to cancer cells. Radioisotopes can be introduced into the body in a way that allows radiation to destroy only the cancerous cells, with little risk to the rest of the body.
Molecules and Compounds
Atoms often bond with each other to form a chemical unit called a molecule. A molecule can contain atoms of the same kind, as when an oxygen atom joins with another oxygen atom to form oxygen gas.
Radioactive substances in the environment can harm cells, damage DNA, and cause cancer. The release of radioactive particles following a nuclear power plant accident can have far-reaching and long-lasting effects on human health. The harmful effects of radiation can also be put to good use, however. Radiation from radioactive isotopes has been used for many years to sterilize medical and dental products. Now the possibility exists that it can be used to sterilize the U.S. mail to free it of possible pathogens, such as anthrax spores. The ability of radiation to kill cells is often applied to cancer cells. Radioisotopes can be introduced into the body in a way that allows radiation to destroy only the cancerous cells, with little risk to the rest of the body.
Molecules and Compounds
Atoms often bond with each other to form a chemical unit called a molecule. A molecule can contain atoms of the same kind, as when an oxygen atom joins with another oxygen atom to form oxygen gas.
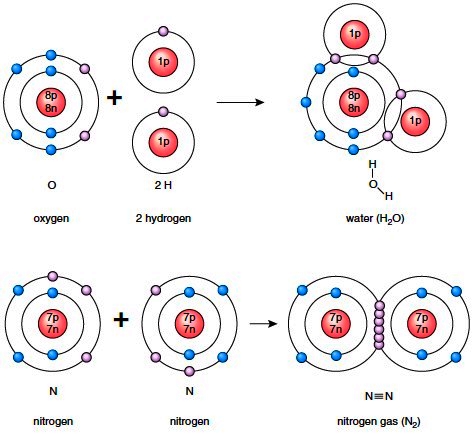
Or the atoms can be different, as when an oxygen atom joins with two hydrogen atoms to form water. When the atoms are different, a compound results. Two types of bonds join atoms: the ionic bond and the covalent bond. The first type of bond can be associated with inorganic molecules, which constitute nonliving matter, and the second type can be associated with organic molecules, which are unique to living things.
Ionic Bonds
Recall that atoms with more than one shell are most stable when the outer shell contains eight electrons. Sometimes during a reaction, atoms give up or take on an electron(s) in order to achieve a stable outer shell. Figure 2.4 depicts a reaction between a sodium (Na) atom and a chlorine (Cl) atom. Sodium, with one electron in the outer shell, reacts with a single chlorine atom. Why? Because once the reaction is finished and sodium loses one electron to chlorine, its outer shell will have eight electrons. Similarly, a chlorine atom, which has seven electrons already, needs only to acquire one more electron to have a stable outer shell. Ions are particles that carry either a positive (+) or negative (-) charge. When the reaction between sodium and chlorine is finished, the sodium ion carries a positive charge because it now has one more proton than electrons, and the chloride ion carries a negative charge because it now has one fewer proton than electrons. The attraction between oppositely charged sodium ions and chloride ions forms an ionic bond. The resulting compound, sodium chloride, is table salt, which we use to enliven the taste of foods. Salts characteristically form an ionic lattice that dissociates in water (Fig. 2.4b). In contrast to sodium, why would calcium, with two electrons in the outer shell, react with two chlorine atoms? Because whereas calcium needs to lose two electrons, each chlorine, with seven electrons already, requires only one more electron to have a stable outer shell. The resulting salt (CaCl2) is called calcium chloride. The balance of various ions in the body is important to our health. Too much sodium in the blood can contribute to hypertension (high blood pressure); not enough calcium leads to rickets (a bowing of the legs) in children; too much or too little potassium results in arrhythmia (heartbeat irregularities). Bicarbonate, hydrogen, and hydroxide ions are all involved in maintaining the acid-base balance of the body.
Ionic Bonds
Recall that atoms with more than one shell are most stable when the outer shell contains eight electrons. Sometimes during a reaction, atoms give up or take on an electron(s) in order to achieve a stable outer shell. Figure 2.4 depicts a reaction between a sodium (Na) atom and a chlorine (Cl) atom. Sodium, with one electron in the outer shell, reacts with a single chlorine atom. Why? Because once the reaction is finished and sodium loses one electron to chlorine, its outer shell will have eight electrons. Similarly, a chlorine atom, which has seven electrons already, needs only to acquire one more electron to have a stable outer shell. Ions are particles that carry either a positive (+) or negative (-) charge. When the reaction between sodium and chlorine is finished, the sodium ion carries a positive charge because it now has one more proton than electrons, and the chloride ion carries a negative charge because it now has one fewer proton than electrons. The attraction between oppositely charged sodium ions and chloride ions forms an ionic bond. The resulting compound, sodium chloride, is table salt, which we use to enliven the taste of foods. Salts characteristically form an ionic lattice that dissociates in water (Fig. 2.4b). In contrast to sodium, why would calcium, with two electrons in the outer shell, react with two chlorine atoms? Because whereas calcium needs to lose two electrons, each chlorine, with seven electrons already, requires only one more electron to have a stable outer shell. The resulting salt (CaCl2) is called calcium chloride. The balance of various ions in the body is important to our health. Too much sodium in the blood can contribute to hypertension (high blood pressure); not enough calcium leads to rickets (a bowing of the legs) in children; too much or too little potassium results in arrhythmia (heartbeat irregularities). Bicarbonate, hydrogen, and hydroxide ions are all involved in maintaining the acid-base balance of the body.

Figure 2.4 Ionic reaction. a. During the formation of sodium chloride, an electron is transferred from the sodium atom to the chlorine atom. At the completion of the reaction, each atom has eight electrons in the outer shell, but each also carries a charge as shown. b. In a sodium chloride crystal, bonding between ions creates a three-dimensional lattice in which each Na+ ion is surrounded by six Cl- ions, and each Cl- is surrounded by six Na+.
Covalent Bonds
As a result of other reactions, atoms share electrons in covalent bonds instead of losing or gaining them. The overlapping outermost shells in Figure 2.5 indicate that the atoms are sharing electrons. Just as two hands participate in a handshake, each atom contributes one electron to the pair that is shared. These electrons spend part of their time in the outer shell of each atom; therefore, they are counted as belonging to both bonded atoms. Covalent bonds can be represented in a number of ways. In contrast to the diagrams in Figure 2.5, structural formulas use straight lines to show the covalent bonds between the atoms. Each line represents a pair of shared electrons. Molecular formulas indicate only the number of each type of atom making up a molecule. A comparison follows:
Structural formula: Cl-Cl
Molecular formula: Cl2
Double and Triple Bonds Besides a single bond, in which atoms share only a pair of electrons, a double or a triple bond can form. In a double bond, atoms share two pairs of electrons, and in a triple bond, atoms share three pairs of electrons between them. For example, in Figure 2.5, each nitrogen atom (N) requires three electrons to achieve a total of eight electrons in the outer shell. Notice that six electrons are placed in the outer overlapping shells in the diagram and that three straight lines are in the structural formula for nitrogen gas (N2). What would be the structural and molecular formulas for carbon dioxide? Carbon, with four electrons in the outer shell, requires four more electrons to complete its outer shell. Each oxygen, with six electrons in the outer shell, needs only two electrons to complete its outer shell. Therefore, carbon shares two pairs of electrons with each oxygen atom, and the formulas are as follows:
Structural formula: O--C--O
Molecular formula: CO2
As a result of other reactions, atoms share electrons in covalent bonds instead of losing or gaining them. The overlapping outermost shells in Figure 2.5 indicate that the atoms are sharing electrons. Just as two hands participate in a handshake, each atom contributes one electron to the pair that is shared. These electrons spend part of their time in the outer shell of each atom; therefore, they are counted as belonging to both bonded atoms. Covalent bonds can be represented in a number of ways. In contrast to the diagrams in Figure 2.5, structural formulas use straight lines to show the covalent bonds between the atoms. Each line represents a pair of shared electrons. Molecular formulas indicate only the number of each type of atom making up a molecule. A comparison follows:
Structural formula: Cl-Cl
Molecular formula: Cl2
Double and Triple Bonds Besides a single bond, in which atoms share only a pair of electrons, a double or a triple bond can form. In a double bond, atoms share two pairs of electrons, and in a triple bond, atoms share three pairs of electrons between them. For example, in Figure 2.5, each nitrogen atom (N) requires three electrons to achieve a total of eight electrons in the outer shell. Notice that six electrons are placed in the outer overlapping shells in the diagram and that three straight lines are in the structural formula for nitrogen gas (N2). What would be the structural and molecular formulas for carbon dioxide? Carbon, with four electrons in the outer shell, requires four more electrons to complete its outer shell. Each oxygen, with six electrons in the outer shell, needs only two electrons to complete its outer shell. Therefore, carbon shares two pairs of electrons with each oxygen atom, and the formulas are as follows:
Structural formula: O--C--O
Molecular formula: CO2
Figure 2.5 Covalent reactions. After a covalent reaction, each atom will have filled its outer shell by sharing electrons. To determine this, it is necessary to count the shared electrons as belonging to both bonded atoms. Oxygen and nitrogen are most stable with eight electrons in the outer shell. Hydrogen is most stable with two electrons in the outer shell.
Contacts: lubopitno_bg@abv.bg www.encyclopedia.lubopitko-bg.com Corporation. All rights reserved.
DON'T FORGET - KNOWLEDGE IS EVERYTHING!