Disorders of Body Fluids
Edema is the accumulation of excessive fluid in the intercellular spaces (Fig. 17-3). Some causes of edema are as follows:
* Interference with normal fluid return to the heart, as caused by congestive heart failure or blockage in the venous or lymphatic systems. A backup of fluid in the lungs, pulmonary edema, is a serious potential consequence of congestive heart failure.
* Lack of protein in the blood. This deficiency may result from protein loss or ingestion of too little dietary protein for an extended period. It may also result from failure of the liver to manufacture adequate amounts of the protein albumin, as frequently occurs in liver disease. The decrease in protein lowers the blood’s osmotic pressure and reduces fluid return to the circulation. Diminished fluid return results in accumulation of fluid in the tissues.
* Kidney failure, a common clinical cause of edema, resulting from the inability of the kidneys to eliminate adequate amounts of urine.
* Increased loss of fluid through the capillaries, as caused by injury, allergic reaction, or certain infections.
Water intoxication involves dilution of body fluids in both the intracellular and extracellular compartments. Transport of water into the cells results in swelling. In the brain, cellular swelling may lead to convulsions, coma, and finally death. Causes of water intoxication include an excess of ADH and intake of excess fluids by mouth or by intravenous injection.
Effusion is the escape of fluid into a cavity or a space. An example is pleural effusion, fluid within the pleural space; in this condition fluid compresses the lung, so that normal breathing is not possible. Tuberculosis, cancer, and some infections may give rise to effusion. Effusion into the pericardial sac, which encloses the heart, may occur in autoimmune disorders, such as lupus erythematosus and rheumatoid arthritis. Infection is another cause of pericardial effusion. The fluid may interfere with normal heart contractions and can cause death.
Ascites is effusion with accumulation of fluid within the abdominal cavity. It may occur in disorders of the liver, kidneys, and heart, as well as in cancers, infection or malnutrition.
Dehydration, a severe deficit of body fluids, will result in death if it is prolonged. The causes include vomiting, diarrhea, drainage from burns or wounds, excessive perspiration, and inadequate fluid intake, as in cases of damage to the thirst mechanism. In such cases, it may be necessary to administer intravenous fluids to correct fluid and electrolyte imbalances.
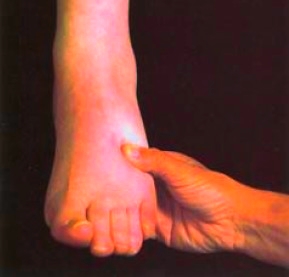
Figure 17-3 Edema of the foot.
Fluid Therapy
This page discussed the rules concerning movement of water into and out of cells when they are placed in different solutions. Recall that an isotonic solution has the same concentration as the cellular fluids and will not cause a net loss or gain of water. A hypertonic solution is more concentrated than cellular fluid and will draw water out of the cells. A hypotonic solution is less concentrated than the cellular fluids and a cell will take in water when placed in this type of solution. These rules must be considered when fluid is administered. Fluids are administered into a vein under a wide variety of conditions to help maintain normal body functions when natural intake is not possible. Fluids are also administered to correct specific fluid and electrolyte imbalances in cases of losses due to disease or injury. The first fluid administered intravenously in emergencies is normal saline, which contains 0.9% sodium chloride, a concentration equal to that of plasma. Because it is isotonic, this type of solution does not change the ion distribution in the body fluid compartments. Frequently, a patient receives 5% dextrose (glucose) in 0.45% (1/2 normal) saline. This solution is hypertonic when infused, but becomes hypotonic after the sugar is metabolized. Another common fluid is 5% dextrose in water. This solution is slightly hypotonic when infused. The amount of sugar contained in a liter of this fluid is equal to 170 calories. The sugar is soon used up, resulting in a fluid that is effectively pure water. Use of these hypotonic fluids is not advisable for long-term therapy because of the common occurrence of water intoxication. Both these dextrose solutions increase the plasma fluid volume. Small amounts of potassium chloride are often added to replace electrolytes lost by vomiting or diarrhea. Ringer lactate solution contains sodium, potassium, calcium, chloride, and lactate. In this formulation, the electrolyte concentrations are equal to normal plasma values. The lactate is metabolized to bicarbonate, which acts as a buffer. This fluid is given when the need is for additional plasma volume with the electrolyte concentration equal to that of the blood. In 25% serum albumin, the concentration of the plasma protein albumin is five times normal. This hypertonic solution draws fluid from the interstitial spaces into the circulation. Fluids containing varied concentrations of dextrose, sodium chloride, potassium, and other electrolytes and substances are manufactured. These fluids are used to correct specific imbalances. Nutritional solutions containing concentrated sugar, protein, and fat are available for administration when oral intake is not possible for an extended period.
This page discussed the rules concerning movement of water into and out of cells when they are placed in different solutions. Recall that an isotonic solution has the same concentration as the cellular fluids and will not cause a net loss or gain of water. A hypertonic solution is more concentrated than cellular fluid and will draw water out of the cells. A hypotonic solution is less concentrated than the cellular fluids and a cell will take in water when placed in this type of solution. These rules must be considered when fluid is administered. Fluids are administered into a vein under a wide variety of conditions to help maintain normal body functions when natural intake is not possible. Fluids are also administered to correct specific fluid and electrolyte imbalances in cases of losses due to disease or injury. The first fluid administered intravenously in emergencies is normal saline, which contains 0.9% sodium chloride, a concentration equal to that of plasma. Because it is isotonic, this type of solution does not change the ion distribution in the body fluid compartments. Frequently, a patient receives 5% dextrose (glucose) in 0.45% (1/2 normal) saline. This solution is hypertonic when infused, but becomes hypotonic after the sugar is metabolized. Another common fluid is 5% dextrose in water. This solution is slightly hypotonic when infused. The amount of sugar contained in a liter of this fluid is equal to 170 calories. The sugar is soon used up, resulting in a fluid that is effectively pure water. Use of these hypotonic fluids is not advisable for long-term therapy because of the common occurrence of water intoxication. Both these dextrose solutions increase the plasma fluid volume. Small amounts of potassium chloride are often added to replace electrolytes lost by vomiting or diarrhea. Ringer lactate solution contains sodium, potassium, calcium, chloride, and lactate. In this formulation, the electrolyte concentrations are equal to normal plasma values. The lactate is metabolized to bicarbonate, which acts as a buffer. This fluid is given when the need is for additional plasma volume with the electrolyte concentration equal to that of the blood. In 25% serum albumin, the concentration of the plasma protein albumin is five times normal. This hypertonic solution draws fluid from the interstitial spaces into the circulation. Fluids containing varied concentrations of dextrose, sodium chloride, potassium, and other electrolytes and substances are manufactured. These fluids are used to correct specific imbalances. Nutritional solutions containing concentrated sugar, protein, and fat are available for administration when oral intake is not possible for an extended period.
Contacts: lubopitno_bg@abv.bg www.encyclopedia.lubopitko-bg.com Corporation. All rights reserved.
DON'T FORGET - KNOWLEDGE IS EVERYTHING!