Proteins
Proteins perform a myriad of functions, including the following:
• Proteins such as collagen and keratin (which makes up hair and nails) are fibrous structural proteins that lend support to ligaments, tendons, and skin.
• Many hormones, which are messengers that influence cellular metabolism, are proteins.
• The proteins actin and myosin account for the movement of cells and the ability of our muscles to contract.
• Some proteins transport molecules in the blood; for example, hemoglobin is a complex protein in our blood that transports oxygen.
• Antibodies in blood and other body fluids are proteins that combine with pathogens or their toxins.
• Enzymes are globular proteins that speed chemical reactions.
Structure of Proteins
Proteins are macromolecules composed of amino acid subunits. An amino acid has a central carbon atom bonded to a hydrogen atom and three groups. The name of the molecule is appropriate because one of these groups is an amino group and another is an acidic group. The third group is called an R group because it is the Remainder of the molecule (Fig. 2.15a). Amino acids differ from one another by their R group; the R group varies from having a single carbon to being a complicated ring structure. When two amino acids join, a dipeptide results; a polypeptide is a chain of amino acids (Fig. 2.15b).

Figure 2.15 Levels of polypeptide structure. a. Amino acids are the subunits of polypeptides. Note that an amino acid contains nitrogen. b. Polypeptides differ by the sequence of their amino acids, which are joined by peptide bonds. c. A polypeptide often twists to become a coil due to hydrogen bonding between members of the peptide bonds. d. The third level of polypeptide structure is due to various types of bonding between the R groups of the amino acids.
Twenty different amino acids are common to polypeptides, which differ by the sequence of their amino acids. The bond between amino acids is called a peptide bond. The atoms of a peptide bond share electrons unevenly; this makes hydrogen bonding possible between members of a polypeptide. Due to hydrogen bonding, the polypeptide often twists to form a coil (Fig. 2.15c). Finally, the coil bends and twists into a particular shape because of bonding between R groups. Hydrogen, ionic, and covalent bonding all occur in polypeptides. Also, any hydrophobic portions of a polypeptide tend to be inside, while the hydrophilic portions are outside where they can make contact with water. Some proteins have only one polypeptide, and others have more than one polypeptide, each with its own so-called primary, secondary, and tertiary structures. If a protein has more than one polypeptide, their arrangement gives a protein a fourth level of structure. The final three-dimensional shape of a protein is very important to its function.When proteins are exposed to extremes in heat and pH, they undergo an irreversible change in shape called denaturation. For example, we are all aware that the addition of acid to milk causes curdling and that heating causes egg white, which contains a protein called albumin, to coagulate. Denaturation occurs because the normal bonding between the R groups has been disturbed. Once a protein loses its normal shape, it is no longer able to perform its usual function. Researchers hypothesize that an alteration in protein organization may be the cause of Alzheimer disease and Creutzfeldt-Jakob disease (the human form of mad cow disease).
Enzymatic Reactions
Metabolism is the sum of all the chemical reactions that occur in a cell. Most cellular reactions will not take place unless an enzyme is present. An enzyme is a protein molecule that functions as an organic catalyst to speed a particular metabolic reaction. Molecules frequently do not react with one another unless they are activated in some way. In the lab, heat is often used to increase the number of effective collisions between molecules. The energy that must be supplied is called the energy of activation. In the body, enzymes lower the energy of activation by forming a complex with particular molecules. In a crowded ballroom, a mutual friend can cause particular people to interact. In a cell, an enzyme brings together certain molecules and causes them to react with one another.
Enzyme-Substrate Complex
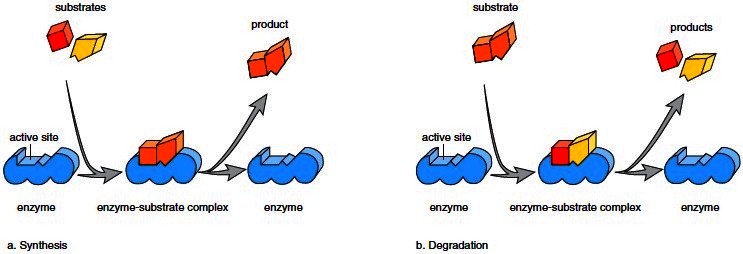
In any reaction, the molecules that interact are called reactants, while the substances that form as a result of the reaction are the products. The reactants in an enzymatic reaction are its substrate(s). Enzymes are often named for their substrate(s); for example, maltase is the enzyme that digests maltose. Enzymes have a specific region, called an active site, where the reaction occurs. An enzyme’s specificity is caused by the shape of the active site, where the enzyme and its substrate(s) fit together, much like pieces of a puzzle (Fig. 2.16). After a reaction is complete and the products are released, the enzyme is ready to catalyze its reaction again:
E+S ® ES ® E+P
(where E = enzyme, S = substrate, ES = enzyme-substrate complex, and P = product).
Metabolism is the sum of all the chemical reactions that occur in a cell. Most cellular reactions will not take place unless an enzyme is present. An enzyme is a protein molecule that functions as an organic catalyst to speed a particular metabolic reaction. Molecules frequently do not react with one another unless they are activated in some way. In the lab, heat is often used to increase the number of effective collisions between molecules. The energy that must be supplied is called the energy of activation. In the body, enzymes lower the energy of activation by forming a complex with particular molecules. In a crowded ballroom, a mutual friend can cause particular people to interact. In a cell, an enzyme brings together certain molecules and causes them to react with one another.
Enzyme-Substrate Complex
In any reaction, the molecules that interact are called reactants, while the substances that form as a result of the reaction are the products. The reactants in an enzymatic reaction are its substrate(s). Enzymes are often named for their substrate(s); for example, maltase is the enzyme that digests maltose. Enzymes have a specific region, called an active site, where the reaction occurs. An enzyme’s specificity is caused by the shape of the active site, where the enzyme and its substrate(s) fit together, much like pieces of a puzzle (Fig. 2.16). After a reaction is complete and the products are released, the enzyme is ready to catalyze its reaction again:
E+S ® ES ® E+P
(where E = enzyme, S = substrate, ES = enzyme-substrate complex, and P = product).
Figure 2.16 Enzymatic action. An enzyme has an active site, where the substrates come together and react. The products are released, and the enzyme is free to act again. a. In synthesis, the substrates join to produce a larger product. b. In degradation, the substrate breaks down to smaller products.
Many enzymes require cofactors. Some cofactors are inorganic, such as copper, zinc, or iron. Other cofactors are organic, nonprotein molecules called coenzymes. Cofactors assist an enzyme and may even accept or contribute atoms to the reaction. It is interesting that vitamins are often components of coenzymes.
Types of Reactions
Certain types of chemical reactions are common to metabolism. Synthesis Reactions: During synthesis reactions, two or more reactants combine to form a larger and more complex product (Fig. 2.16a). The dehydration synthesis reaction we have already studied (i.e., the joining of subunits to form a macromolecule) is an example of a synthesis reaction. When glucose molecules join in the liver, forming glycogen, a synthesis reaction has occurred. Notice that synthesis reactions always involve bond formation and therefore an input of energy. Degradation Reactions: During degradation reactions, a larger and more complex molecule breaks down into smaller, simpler products (Fig. 2.16b). The hydrolysis reactions that break down macromolecules into their subunits are examples of degradation reactions, also called decomposition reactions. When protein is digested to amino acids in the stomach, a degradation reaction has occurred. Replacement Reactions: Replacement reactions involve both degradation and synthesis. For example, when ADP joins with inorganic phosphate, "P" , and ATP forms, the last hydrogen in ADP is replaced by a "P" (see Fig. 2.18). The "P" loses a hydroxyl group. The hydrogen and hydroxyl group join to become water.
Types of Reactions
Certain types of chemical reactions are common to metabolism. Synthesis Reactions: During synthesis reactions, two or more reactants combine to form a larger and more complex product (Fig. 2.16a). The dehydration synthesis reaction we have already studied (i.e., the joining of subunits to form a macromolecule) is an example of a synthesis reaction. When glucose molecules join in the liver, forming glycogen, a synthesis reaction has occurred. Notice that synthesis reactions always involve bond formation and therefore an input of energy. Degradation Reactions: During degradation reactions, a larger and more complex molecule breaks down into smaller, simpler products (Fig. 2.16b). The hydrolysis reactions that break down macromolecules into their subunits are examples of degradation reactions, also called decomposition reactions. When protein is digested to amino acids in the stomach, a degradation reaction has occurred. Replacement Reactions: Replacement reactions involve both degradation and synthesis. For example, when ADP joins with inorganic phosphate, "P" , and ATP forms, the last hydrogen in ADP is replaced by a "P" (see Fig. 2.18). The "P" loses a hydroxyl group. The hydrogen and hydroxyl group join to become water.
Figure 2.18 ATP reaction. ATP, the universal energy currency of cells, is composed of adenosine and three phosphate groups (called a triphosphate). When cells require energy, ATP undergoes hydrolysis, producing ADP "P" , with the release of energy. (The "P" stands for inorganic phosphate.) Later, ATP is rebuilt when energy is supplied and ADP joins with "P".

Contacts: lubopitno_bg@abv.bg www.encyclopedia.lubopitko-bg.com Corporation. All rights reserved.
DON'T FORGET - KNOWLEDGE IS EVERYTHING!